Graphene Band Structure Explained
Graphene is known as a very stable material. In fact, studies show that this nearly-transparent material is by weight has more strength than steel by 207 times. It efficiently conducts electricity and heat as well. Two-dimensional properties of carbon crystalline allotrope make up graphene.
The graphene band structure looks like a hexagonal pattern of chicken wire with carbon atoms that are packed densely in an atomic scale. Each carbon atom of the graphene band structure is made up of four bonds Three neighbors of one σ bond along with itself and oriented out of plane is the π-bond.
These carbon atoms’ distance from each other is about 1.42 Å. There are two triangular lattices that are interleaving that may be regarded on the hexagonal lattice of graphene. Using this perspective, the graphene band structure was calculated successfully on one layer of graphite with the use of tight-binding approximation.
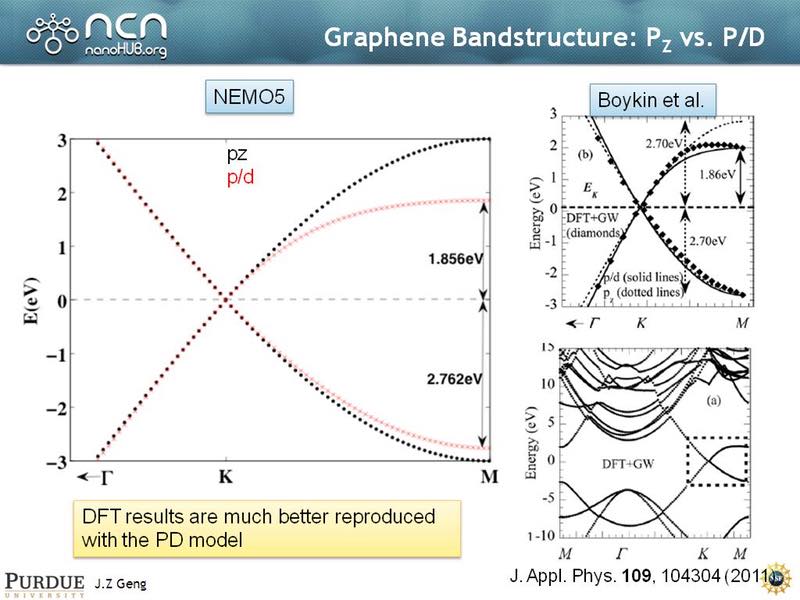 Graphene Band Structure
Graphene Band Structure
The Graphene Band Structure Is Stable
This is because the carbon atoms are packed tightly. Aside from that, its stability can be attributed to the orbital s combinations, px and py that make up the σ bond otherwise known as the sp2 orbital hybridization. The π-bond is made up of the final pz electron.
When hybridized together, π-bond form π*-bands and π-band, through a half-filled band that allows electrons that are free-moving. Evidence of the diffraction for the layering of graphite is shown in the solid form of graphene sheets. This can be seen in some nano-structures that are single-walled.
However, in the pre-solar graphite onions’ core, graphene with no layers but rings alone can be found. Studies done using transmission electron microscope or TEM on flat sheets of graphene appear faceting defects. From a melt, two-dimensional crystallizations play a role as well according to the same studies.
To Further Prove The Stability Of Graphene Band Structure
Studies have proven that if there are holes within graphene sheets, graphene can self-repair these holes if hydrocarbons or other carbon-containing molecules are exposed to it.
When exposed to pure carbon atoms, these defects or holes are fixed and filled completely as perfect hexagons are formed by the atoms. There are also TEM studies on the atomic structure of single-layer graphene that are isolated on graphene sheets that are suspended between metallic grid bars.
Honeycomb lattice are shown on electron diffraction patterns. Flat sheet “rippling” is also shown on graphene that is suspended with approximately one-nanometre amplitude. Because of the unstable two-dimensional crystals, the ripples on graphene may be intrinsic to the material. It may also come from ubiquitous dirt as seen on graphene images from TEM studies.